|
� |
| |
Information | |
Introduction, History and Evolution and Organization
is described under the section on General Information. In this section, the practical
aspects of COPCORD are described. COPCORD does not have a firm policy document
on this subject. The description is based on personal experience and knowledge
(AC, Bhigwan India) and interactions with several COPCORD investigators. The reader
is advised to read COPCORD publications
(see another section in this website) to learn more. There are three stages to
a COPCORD. The first stage comprises of three phases which are best completed
concurrently.
| | STAGE
I – Community Survey
| | | |
PHASE 1:
Identify Cases past/current
PHASE 2:
Record Symptoms, Disability, Function And Perceptions
PHASE
3: Rheumatology Evaluation
| Note:
Investigations (lab and X-Rays) have been carried out in selected cases as a component
of Phase 3 in majority of the surveys. However, investigations have been featured
as Phase 4 by some surveys.
| | |
STAGE II: Identify Risk Factors and Incidence Cases, Impact Health
Education.
STAGE III: Control and Prevention Strategy,
Improved Health Care
| Pre-requisite:
Who should lead a COPCORD? Though any doctor with an interest in musculoskeletal
disorders and community medicine can conduct a COPCORD, a rheumatologist is best
suited. Some prior fundamental knowledge about epidemiology and biostatistics
is required especially if COPCORD Stages II and III are to be carried out. Though
the program was conceived by WHO ILAR collaboration, permission is not required
from any organization or institution. It is advisable to liaise with ILAR (International
League of Associations for Rheumatology) COPCORD coordinator to obtain information
and guidance. If the COPCORD investigator belongs to an APLAR (Asia Pacific League
of Associations for Rheumatology) country, guidance can also be obtained from
the APLAR COPCORD co-ordinator. Also, in the past, seed money grant was provided
to the COPCORD investigator by ILAR/APLAR and the survey was monitored by an independent
referee who was generally appointed by ILAR/APLAR Co-ordinator. However, due to
certain re-organization in recent years, ILAR/APLAR have discontinued the practice
of granting seed money. A referee experienced in COPCORD can be of critical assistance
to an investigator beginning a maiden program. There are several issues of data
collection which need to be addressed (best with the referee) so that the results
of survey are robust (based on standard methods), reliable, meaningful and comparable
(to other COPCORD surveys). The referee can be of immense help to prepare and
present/publish results in peer reviewed journals.
Area:
The majority of completed surveys have been in a non randomized site selected
by preference. In the latter case, a well defined geographical area (village or
a town suburb) should be chosen. The latter could be driven by several important
local factors ranging from ease of survey, easy access, co-operative administration
and medical set up. However, for better representation, a randomized method for
selection of COPCORD site is recommended. It should be remembered that the logistics
of a randomized method are more difficult, cumbersome and challenging. Does the
end result differ significantly? Logically, differences can be expected but nobody
has carried such comparable studies for musculoskeletal pain and disorders (MSK).
As COPCORD aims to identify common MSK in a community, the investigator should
not hesitate to adopt a non randomized selection in the interest of a quick socioeconomically
feasible survey.
Depending upon the economics and logistics, the investigator
may choose an urban and a rural site in a region for better capture of spectrum
and extent of MSK. While choosing sites, the issue of ‘migration and temporary
inhabitants’ should also be kept in mind.
Population size: The size depends
upon the expected prevalence of MSK in the community. Again, the search is for
common disorders and not rare disorders like lupus and vasculitis. The current
practice is to have a sampling frame size of about 3500-4000 population. If the
sample size is less than 2500, the prevalence estimates of important inflammatory
disorders like rheumatoid arthritis and seronegative spondyloarthritis may be
underestimated.
Team: COPCORD being a community program requires a well
balanced multidiscipline team that gels well and works under a dynamic and encouraging
leadership. Though the leadership is medical, the backbone of success lies with
the paramedical (PM) staff and health workers (HW) who go from house to house
during the survey. Besides a rheumatologist, medical graduates are required to
carry out screening and related activities. A local community leader, preferably
a doctor (from the COPCORD site), who knows the community well and is respected
(by the community) should be identified well in advance and made a member of the
team to advise and help co-ordinate the various survey activities. A PM/clinical
research personnel with some experience in administration and finance can work
as a ‘team manager/coordinator’. Who is a HW? Surveys in the past have used different
personnel as HW-nurses, trained medical social workers and volunteers. HW volunteers
should be trained in a focused workshop of 1-2 days on the methods required to
carry out the survey, complete questionnaires and assist doctors. Educated young
enthusiastic trained volunteers from the community and working under expert medical
supervision/coordination can be of special value to complete surveys expeditiously.
The team will also need laboratory technicians to collect blood samples. Personnel
will also be required to enter survey data. A biostatistician, probably part time,
can be a great asset and if feasible should be involved at the protocol writing
stage much prior to beginning survey.
A special modest dress code or
even a single unique dress item (shirt/
cap) for the team can go a long way to suffuse a sense of commitment and pride.
Protocol & Ethical Issues: A protocol should be prepared as a comprehensive
document describing the site selection and population size, planned activities
of the different phases of the survey, personnel involved and their job descriptions,
planned investigations, time table/schedule of events, questionnaires
and case record forms used, glossary of terms and classification
of disorders and diseases.
A community survey should be treated
like a precise laboratory experiment which must be completed in time in a proper
manner. Unlike a laboratory scenario, a survey involves much more human involvement
and movement and encounters several unpredictable events and happenings. An astute
investigator can try and fore see as many unpredictable events and variables and
plan well in advance. This requires personal experience but more of interacting
and seeking advice from peers, seniors, experts and local community leaders/doctors.
The time table/schedule of events with reference to survey phases should be practical
and binding on all concerned personnel.
The protocol should be reviewed
by the institutional scientific/ethic committee. Advice should be explicitly obtained
on issues of ethic and patient consent. This is important if blood samples are
to be taken from survey subjects and further stored for future investigations.
Questionnaires (Qs): These are the
heart and soul of the survey. The current Qs are described in a separate section
and can be downloaded. Sufficient time should be spent on finalizing the Qs prior
to beginning the survey.
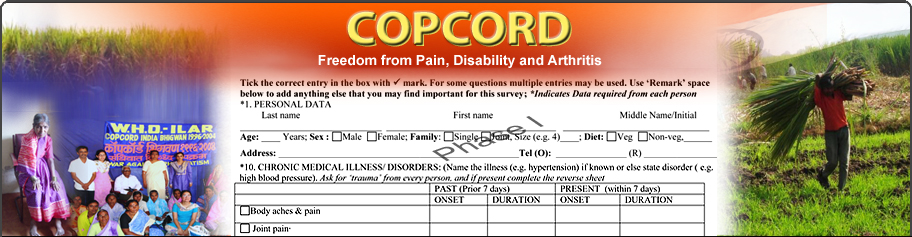
Phase 1 Qs:
The aim is to identify respondents with current/past MSK pain who will be further
assessed in phase 2. For easy comprehension, the questions can specifically ask
for presence of joint and/or soft tissue pains. The Qs also collects general demographic
data including occupation, religion and habits. Tobacco and trauma are important
plausible risk factors and enough information should be collected. This phase
also captures the self reported co-morbidity and this data can effectively demonstrate
the overall supreme position of MSK burden in the community (as has been published
from Pune Bhigwan India studies).
Phase
2 Qs: The respondents volunteer detail information about their pain and MSK
in this phase. Pain sites, past (prior to seven days) and present, are preferably
marked on a human mannequin. The impact of pain/MSK as perceived by the respondent
can be included. It is recommended that this phase also includes some measurement
of impact on quality of life (QOL) using globally accepted and validated instruments
like HAQ (health assessment questionnaire) and WHO-QOL. Data on local resources
of treatment, including popular traditional medicinal practices, is also obtained.
Phase 3 Qs: This is a typical rheumatology
case record form (CRF). As COPCORD is largely driven by clinical cues and expertise,
the CRF must be strongly oriented towards capturing clinical events and profile.
A striking feature of all surveys completed till date have been the preponderance
of non specific arthralgias and soft tissue aches and pains suggestive of some
kind of a soft tissue rheumatism (not necessarily fibromyalgia) which are difficult
to classify as per the norms of International Classification of Diseases (ICD).
Several of the latter may be related to trauma or occupational overuse. To overcome
such difficulties, Bhigwan India COPCORD came up with a ‘clinical
classification of MSK disorders’ that would capture maximum information on
NSA and STR and ensure comparability. The latter classification has since then
been used in several COPCORD surveys. COPCORD core questionnaires (CCQ) have undergone
an evolution as per existing experience and rheumatology knowledge. From very
cumbersome lengthy Qs, they have been reduced to a computer data friendly capture
of core information (enunciated at inception). The COPCORD investigator is advised
to maintain the design of the phases and their core questions but has the freedom
to add any more data questions that may be relevant and appropriate to the local
scenario. The final CCQ to be used in the survey need to be translated into the
local language and validated through pilot testing in the community (healthy subjects
and subjects suffering from MSK and other disorders). In a similar vein, the investigator
can use the regional rheumatology CRF as far as disorders defined clinically are
captured. The latter does not prevent the use of internationally accepted classification
criteria.
How should the data be recorded in Qs? Should the subjects
enter the data or should it be filled through interviews by the HW. There are
disparate opinions on this subject in the COPCORD literature. Overall, the surveys
have followed what was comfortable to the community. But this should be decided
a-priori. Most of the surveys have allowed HW to complete the Qs in a face-face
interview. It cannot be emphasized enough that the HW should not influence the
answers of the community. The latter is a difficult proposition and is often neglected
but should be adequately addressed by the investigator with the HW in their pre-survey
training workshop.
Finance & Sponsorship: Unfortunately, there are no
central COPCORD funds available with either ILAR or APLAR at this point of time.
Earlier a seed grant was provided to the investigator. However, a prospective
investigator is advised to be in touch with these central agencies because newer
organizations often have a different perspective on this subject. And funding
COPCORD studies is a very critical proposition to help rheumatology find roots
in the several developing economies in Asia, Africa and Latin America.
COPCORD is about local thinking and regional resources. The majority of COPCORD
investigators who have completed surveys till date have managed funds from regional
philanthropic agencies with nil or minimal support from the pharmaceutics. The
experts including rheumatologists have usually volunteered their time and expertise.
But the rest of the team needs to be well paid. Finance planning also includes
logistics (especially travel to COPCORD site), diagnosis investigations and ancillary
services (data entry and statistical analysis). If the services of a referee or
outside advisor are requisitioned, an additional budget will be required.
COPCORD survey is a socioeconomically appealing model and none should be
dissuaded by the challenge of raising funds to complete the task. BJD India recently
raised central funds from their corporate partners (pharma sector) to fund COPCORD
surveys in 12 sites all over India. Not much has been published on the cost of
COPCORD.. The expenditure of the first three years of Bhigwan India COPCORD was
less than USD2 per person in the village (Clin Rheumatol 2006, 25: 443-7).
Pre-survey Activities: Besides planning, training and rehearsal, and translating
key Qs, there are several other important activities that an investigator and
key personnel can undertake prior to the survey. The community and local doctors
in particular needs to be well informed about the upcoming survey. Meetings should
be held with community leaders and medical fraternity. The latter must include
local administration. Distribution of some basic information in small pamphlets
by conducting a rally just a day or two prior to the beginning of the survey may
be useful to sensitize the community. Central site should be chosen for Phase
3 rheumatology examination of respondents. As rheumatology is a sub served specialty,
continuing medical education program can be conducted for the local/regional doctors
and COPCORD introduced. The local media should be involved in providing information
to the community. The COPCORD team should carry out a thorough reconnaissance
of the COPCORD site. Also, information on the community KAP (knowledge, attitude
and practices) should be obtained which can be of immense help in planning survey
and preparing protocol.
It may also be extremely useful for the investigator
to visit a completed COPCORD survey site and obtain first hand practical information.
Prior to initiating national COPCORD surveys at 12 sites, BJD India sponsored
several 2 day COPCORD training workshops on a regional basis for the new investigators.
The latter was conducted by senior and experienced COPCORD investigators.
Conducting Survey: This should be like a military operation where each personnel
know what he or she is expected to do within the time frame. The investigator
should chalk out the entire program using time lines and geographical sketches
and thoroughly discuss the plan and strategy with the entire team. The site can
be divided into multiple zones. Each zone can be allotted a team of 2-3 HW; some
for Phase 1 and other for Phase 2 so that the process continues smoothly and non-respondents
are reduced to minimum. The HW must try and track down the potential respondent
on at least 3 different days and time before declaring a non-respondent. Each
team should have a team leader and given a realistic daily target for completion
of Phase 1 and 2 Qs. Every day, a count of Qs completed by each HW should be done
by the team manager prior to disbursing daily remuneration. This ensures accountability
and check of the quality of completed Qs. The medical team should begin their
Phase 3 operation once enough Phase 2 respondents have been identified. In other
words, all the three phases of the survey must proceed concurrently. Some of the
subjects may require more than one visit to complete clinical evaluation. An easily
accessible site in the community (a community hall, school or a Government run
dispensary) should be chosen for Phase 3 evaluation. Private medical centers should
be avoided. The medical team may have to make house visits to examine the very
disabled and non ambulant subjects. A good coordination is required between HW
and medical team to ensure speedy and timely evaluation of Phase 2 respondents.
Community surveys cannot linger on. They must move speedily in a well
coordinated smooth manner. This ensures the enthusiasm of the community and the
COPCORD team. Remember the community is also watching and assessing the survey.
It will provide better support if the team displays a high level of commitment
and serious professional performance. Every team member must work with enthusiasm
to help the subject eventually feel and get better. The community must stand benefited
from the survey and in this regard the medical team has to devote enough attention
to each respondent in trying to understand the MSK problem and advise remedy.
The community is not all that interested in the academics and data of the survey.
It seeks attention from the medical faculty and relief from the MSK pain and disability.
But above all, the scientific merit of the survey data improves if it is completed
thoroughly and expeditiously. Acute events can be properly observed and captured.
There are less non respondents. As the doctors work neck to neck with the HW,
the team spirit remains high. Finally, this leads to better logistics and economics.
It makes sense to employ a sufficiently large number of HW to spread quickly in
the community from the time ‘go’ and acquire data. The investigator and the survey
co-ordinator/manager must review the tally of Phases 1, 2 & 3 on a regular basis
and collate the numbers with the population sample size target and timelines to
identify any lag in the survey or lack of respondents.
Lab & Imaging
& Special Investigations: COPCORD is not an investigation intensive program. But
a certain amount of investigations would be required in modern times to make sensible
diagnosis of some of the myriad aches and pains that community suffers from. The
extent of investigations would be dictated by the index of clinical suspicion
and the budgetary allocation. But investigators can plan to preserve sera in deep
freeze for future investigations. Blood samples need to be collected soon after
the clinical examination is over at the Phase 3 site. Simple procedures like erythrocyte
sedimentations rate and hemoglobin measurement, making peripheral smears, dipstick
urine analysis and serum separation can be organized at the Phase 3 site itself.
X-rays invariably means travel and HW would have to ensure that subjects are not
unnecessarily inconvenienced when asked to get an X-ray done.
Data Entry:
It is prudent to add that data entry must begin as soon as possible after the
survey has progressed sufficiently. This ensures good communication between HW
and data entry operators in case of ineligible data or missing data. A Microsoft
excel sheet is good enough for data entry in case a special soft ware (based on
Qs in use) is not available.
Longitudinal Phase: Stages II and III required
special expertise of community and preventive medicine, public health, medical
social sciences, epidemiology and biostatistics. It is prudent and practical to
add that this is an expensive phase and requires prolonged dedication by the investigator
and the team. Also, the community needs to be sensitized in a special manner.
Inception cohort studies can be carried out to study and determine the natural
history and risk determinants. Case (or even nested case) control studies can
evaluate risk factors. Health education should be based on Stage I data and various
popular community methods can be employed. In fact, basic health education on
MSK pain and disorders should be imparted during Phase 3 of survey by trained
team personnel and doctors. Repeat surveys can be carried out in time to determine
the impact of COPCORD.
Rheumatology Care Services: COPCORD was not meant
to provide rheumatology treatment care services. Encouraged by the community and
concerned with the woeful lack of basic rheumatology care in the region, Bhigwan
India COPCORD for the first time provided free of cost rheumatology consultations
to the patients and conducted intensive CME programs for the local doctors. The
local doctors were taught how to handle rheumatology problems in general practice.
The latter was singularly responsible for an overwhelming community support and
enthusiasm that drove the Bhigwan COPCORD into Stages II and III and expanded
the COPCORD coverage to almost 200 villages with a combined population exceeding
200,000. Bhigwan COPCORD is currently in its 15th year of operation. All this
has led to much discussion amongst COPCORD investigators on the subject but there
is no consensus on whether COPCORD should extend its services. Therefore, it is
not within the COPCORD ambit at this point of time to provide rheumatology treatment
services although it is a laudable objective to consider. | | | | |
|